Short Answer
There are ________ mol of bromide ions in 0.500 L of a 0.300M solution of Al 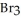 .
.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: Identify the polyprotic acid.<br>A) H<sub>2</sub>SO<sub>4</sub><br>B) HCl<br>C) LiI<br>D)
Q54: Give the net ionic equation for the
Q66: A FeCl<sub>3</sub> solution is 0.175 M.How many
Q68: What is the molarity of a potassium
Q69: When dissolved in water,RbOH behaves as<br>A) an
Q72: How many grams of C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6105/.jpg"
Q74: What is the concentration of HCl in
Q75: Which of the compounds H<sub>3</sub>PO<sub>4</sub> <sub> </sub>,Mg(OH)<sub>2</sub>,LiOH,and
Q119: The titration of 80.0 mL of an
Q220: How many sodium ions are present in