Multiple Choice
Based on the balanced chemical equation shown below,determine the mass percent of Fe3+ in a 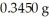 sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
sample of iron ore,if 22.40 mL of a 0.1000 M stannous chloride,SnCl2(aq) ,solution is required to completely react with the Fe3+ present in the ore sample.The chemical equation for the reaction is 2 Fe3+(aq) + Sn2+(aq) → 2 Fe2+(aq) + Sn4+(aq) .
A) 11.59%
B) 18.13%
C) 36.26%
D) 72.52%
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Give the net ionic equation for the
Q112: Give the name for HNO<sub>2</sub>.
Q117: Based on the balanced chemical equation shown
Q118: Explain the difference between a strong and
Q156: Identify sugar.<br>A) strong electrolyte, weak acid<br>B) weak
Q157: How many of the following compounds are
Q158: How many H<sup>+</sup> ions can the acid,H<sub>2</sub>SeO<sub>4</sub>
Q160: Which of the following compounds is insoluble
Q162: Balance the chemical equation given below,and determine
Q163: Determine the concentration of a solution prepared