Multiple Choice
Which of the compounds of H CO2H , Ba(OH) 2,CsOH,and H Br,behave as acids when they are dissolved in water?
A) Ba(OH) 2 and CsOH
B) 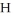
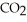 H and H Br
H and H Br
C) only H Br
D) only CsOH
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q95: Identify the oxidation state of H in
Q101: A solution is prepared by mixing 20.0
Q102: How many moles of Na F are
Q103: Identify the oxidation state of H in
Q105: What is the oxidation number change for
Q108: What is the oxidation number of the
Q110: What is the concentration (M)of a NaCl
Q111: Calculate the number of grams of solute
Q172: Determine the reducing agent in the following
Q186: Which of the following is an acid