Multiple Choice
A solution is prepared by adding 1.50 g of solid NaCl to 50.0 mL of 0.100 M 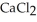 .What is the molarity of chloride ion in the final solution? Assume that the volume of the final solution is 50.0 mL.
.What is the molarity of chloride ion in the final solution? Assume that the volume of the final solution is 50.0 mL.
A) 0.713
B) 0.613
C) 0.130
D) 0.230
E) 0.513
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Give the net ionic equation for the
Q118: Explain the difference between a strong and
Q128: Identify acetic acid.<br>A) strong electrolyte, weak acid<br>B)
Q160: Which of the following compounds is insoluble
Q162: Balance the chemical equation given below,and determine
Q163: Determine the concentration of a solution prepared
Q166: Which one of the following compounds is
Q167: Which of the following pairs of aqueous
Q168: What reagent could be used to separate
Q169: Which of the following pairs of aqueous