Multiple Choice
Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains 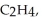 flask B contains O3,and flask C contains F2.Which flask contains the largest number of molecules?
flask B contains O3,and flask C contains F2.Which flask contains the largest number of molecules?
A) flask A
B) flask B
C) flask C
D) All contain same number of molecules.
Correct Answer:

Verified
Correct Answer:
Verified
Q22: Determine the volume of SO<sub>2</sub> (at STP)formed
Q55: Which of the following will cause the
Q63: Which of the following statements is TRUE?<br>A)
Q69: Given the equation C<sub>2</sub>H<sub>6</sub>(g)+ O<sub>2</sub>(g)→ CO<sub>2</sub>(g)+ H<sub>2</sub>O(g)(not
Q72: A mixture of N<sub>2</sub>,O<sub>2</sub> and He have
Q84: Why does hot air rise?
Q111: The mole fraction of argon in dry
Q116: The density of chlorine gas at 1.21
Q124: Determine the total volume of all gases
Q179: A 0.334 g sample of an unknown