Multiple Choice
A 1.44-g sample of an unknown pure gas occupies a volume of 0.335 L at a pressure of 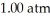 and a temperature of 100.0°C.The unknown gas is
and a temperature of 100.0°C.The unknown gas is
A) argon
B) helium
C) krypton
D) neon
E) xenon
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q7: In the laboratory,hydrogen gas is usually made
Q17: A syringe initially holds a sample of
Q29: Given three cylinders containing O<sub>2</sub> gas at
Q48: Which of the following gas samples would
Q49: Given the equation C<sub>2</sub>H<sub>6</sub>(g)+ O<sub>2</sub>(g)→ CO<sub>2</sub>(g)+ H<sub>2</sub>O(g)(not
Q50: An instrument used to measure atmospheric pressure
Q57: Which of the gases in the graph
Q64: A 2.75-L container filled with CO<sub>2</sub> gas
Q109: The total pressure of a gas mixture
Q149: What pressure would a gas mixture in