Multiple Choice
Consider the phase diagram shown.What is the normal freezing point? 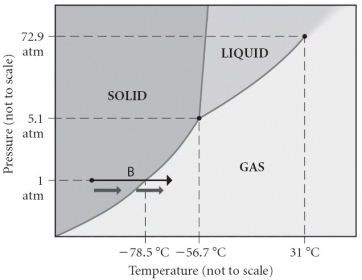
A) 31°C
B) -56.7 °C
C) -78.5 °C
D) 0°C
E) 100°C
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which substance below has the strongest intermolecular
Q6: Place the following compounds in order of
Q40: Which has the smallest dipole-dipole forces?<br>A)CH<sub>3</sub>Cl<sub> </sub><br>B)HBr<sub>
Q60: Choose the compound that exhibits hydrogen bonding
Q64: Identify the place which has the highest
Q66: Choose the pair of substances that are
Q67: Identify the substance with the highest viscosity.<br>A)
Q68: The melting point of water is<br>A) 32<sup>o</sup>F<br>B)
Q70: Identify the compound that has hydrogen bonding.<br>A)
Q98: At atmospheric pressure,dry ice<br>A) freezes.<br>B) deposits.<br>C) sublimes.<br>D)