Multiple Choice
Consider the phase diagram below.If the dashed line at 1 atm of pressure is followed from 100 to 500°C,what phase changes will occur (in order of increasing temperature) ? 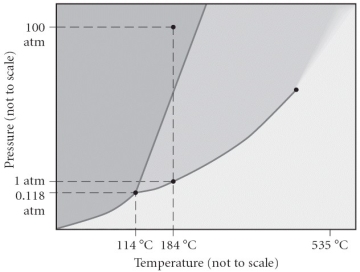
A) condensation, followed by vaporization
B) sublimation, followed by deposition
C) vaporization, followed by deposition
D) fusion, followed by vaporization
E) No phase change will occur under the conditions specified.
Correct Answer:

Verified
Correct Answer:
Verified
Q4: The boiling point of water is<br>A) 0<sup>
Q5: Which of the following must be overcome
Q49: Identify the characteristics of a liquid.<br>A) indefinite
Q57: How much heat is released when 105
Q59: Sketch the phase diagram of benzene.Make sure
Q90: The normal boiling point for H<sub>2</sub>Se<sub> </sub>
Q94: The enthalpy change for converting 10.0 g
Q97: Determine the vapor pressure (in torr)of a
Q103: How much energy must be removed from
Q121: Define viscosity.