Multiple Choice
The fluorocarbon C2CL3F3 has a normal boiling point of 47.6°C.The specific heats of C2CL3F3(1) and C2CL3F3(g) are 0.91 J/gK and 0.67 J/gK,respectively.The heat of vaporization of the compound is 27.49 kJ/mol.The heat required to convert 50.0 g of the compound from the liquid at 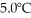 to the gas at 80.0°C is ________ kJ.
to the gas at 80.0°C is ________ kJ.
A) 8.19
B) 1454
C) 30.51
D) 3031
E) 10.36
Correct Answer:

Verified
Correct Answer:
Verified
Q28: Define fusion.<br>A) the phase transition from solid
Q53: The forces between polar molecules is known
Q64: Determine the normal boiling point of a
Q95: Place the following substances in order of
Q99: Identify the compound that does not have
Q102: An unknown substance has a boiling point
Q103: The boiling point of H<sub>2</sub>O is much
Q104: Choose the pair of substances that are
Q105: Choose the substance with the highest viscosity.<br>A)
Q110: Choose the pair of substances that are