Multiple Choice
Consider the phase diagram shown below.If you start at 0.75 atm and 0 °C,and move to 0.10 atm and 25C,you will move from the ________ phase to the ________ phase. 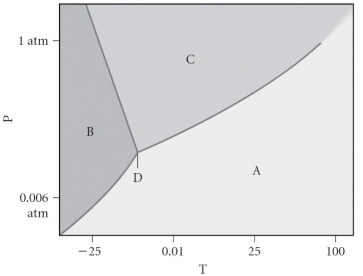
A) liquid, gas
B) gas, gas
C) liquid, liquid
D) gas, solid
E) liquid, solid
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Define deposition.<br>A) A liquid becomes a gas.<br>B)
Q39: How much energy is required to vaporize
Q47: How much energy is required to vaporize
Q66: What is the strongest type of intermolecular
Q102: Identify the compound that does not have
Q114: The freezing point of water is<br>A) 32<sup>o</sup>F<br>B)
Q115: Consider the phase diagram shown.Choose the statement
Q118: Based on the figure above,the boiling point
Q120: Which of the following compounds has dipole-dipole
Q122: Calculate the total quantity of heat required