Multiple Choice
What is the hydronium ion concentration and the pH for an aqueous solution of NH3 that has a hydroxide ion concentration of 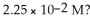
A) 4.44 × 10-12 M and 2.65
B) 4.44 × 10-12 M and 11.35
C) 4.44 × 10-13 M and 1.65
D) 4.44 × 10-13 M and 12.35
Correct Answer:

Verified
Correct Answer:
Verified
Q29: Determine the pH in a 0.235 M
Q61: Do both protons ionize instantaneously from a
Q81: What is the concentration of hydroxide ions
Q85: Calculate the concentration of H<sub>3</sub>O⁺ in a
Q96: What is the conjugate acid of HCO<sub>3</sub>⁻
Q125: Calculate the pH of a 1.60 M
Q126: Which of the following is a Brønsted-Lowry
Q131: Determine the [OH<sup>-</sup>] concentration of a 0.153
Q133: Place the following in order of increasing
Q135: Identify the diprotic acid.<br>A) HNO<sub>3</sub><br>B) HBr<br>C) CH<sub>3</sub>COOH<br>D)