Multiple Choice
What is the hydronium ion concentration of a 0.150 M hypochlorous acid solution with 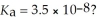 The equation for the dissociation of hypochlorous acid is:
The equation for the dissociation of hypochlorous acid is: 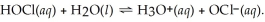
A) 1.9 × 10-4 M
B) 7.2 × 10-4 M
C) 2.8 × 10-5 M
D) 7.2 × 10-5 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: Determine the pH of a 0.461 M
Q33: What is the autoionization of water?
Q40: Which of the following is a polyprotic
Q46: Which of the following solutions would have
Q58: What is the difference between a strong
Q59: Give the characteristics of a strong acid.<br>A)
Q64: In a triprotic acid,which K<sub>a</sub> has the
Q72: Calculate the pH of a solution that
Q97: Which of the following bases is the
Q117: Which of the following is a weak