Multiple Choice
Calculate the pH of a 0.80 M H2SO3,solution that has the stepwise dissociation constants Ka1 = 1.5 × 10-2 and 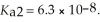
A) 0.96
B) 0.99
C) 1.82
D) 1.92
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q113: Calculate the hydroxide ion concentration in an
Q113: Determine the K<sub>a</sub> of an acid that
Q114: Determine the pH of a 0.232 M
Q117: The base-dissociation constant of ethylamine (C<sub>2</sub>H<sub>5</sub>NH<sub>2</sub>)is 6.4
Q120: Identify the food that is not acidic.<br>A)
Q122: Calculate the pH of a 0.020 M
Q123: What is the pH of a 0.84
Q127: Determine the pOH of a 0.00598 M
Q155: Determine the pH of a 0.188 M
Q170: When dissolved in water,which compound is generally