Multiple Choice
What is the pH of a 0.50 M H2Se solution that has the stepwise dissociation constants Ka1 = 1.3 × 10-4 and 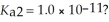
A) 2.09
B) 3.89
C) 4.19
D) 5.57
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q40: Which of the following acids will have
Q88: Identify the acid that is in vinegar.<br>A)
Q89: Calculate the hydronium ion concentration in an
Q90: A Lewis acid<br>A) donates electrons.<br>B) accepts electrons.<br>C)
Q93: All of the following anions are considered
Q94: What is the hydroxide ion concentration and
Q96: Identify the acid that is in car
Q118: Determine the K<sub>b</sub> for CN⁻ at 25°C.The
Q161: Which one of the following salts,when dissolved
Q179: Which one of the following salts,when dissolved