Multiple Choice
Calculate the pH of a solution that is 0.111 M in sodium formate (NaHCO2) and 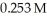 in formic acid (HCO2H) .The Ka of formic acid is 1.77 × 10-4.
in formic acid (HCO2H) .The Ka of formic acid is 1.77 × 10-4.
A) 3.387
B) 4.103
C) 14.36
D) 10.61
E) 5.296
Correct Answer:

Verified
Correct Answer:
Verified
Q13: Calculate the pH of a solution formed
Q42: Formic acid (HCO<sub>2</sub>H,K<sub>a</sub> = 1.8 × 10<sup>-4</sup>)is
Q88: A 100.0 mL sample of 0.20 M
Q120: Describe the information necessary (and why)to chose
Q121: The molar solubility of Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> is 8.89
Q122: What is the molar solubility of AgCl
Q123: A 25.0 mL sample of 0.150 M
Q125: You wish to prepare an HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub> buffer
Q126: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS,AgCl,NH<sub>4</sub>Cl,and ZnS.Identify the
Q128: What is the molar solubility of Mg(OH)<sub>2</sub>