Multiple Choice
Calculate the pH of a solution that is 0.210 M in nitrous acid ( 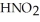 ) and 0.290 M in potassium nitrite (
) and 0.290 M in potassium nitrite ( 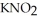 ) .The acid dissociation constant of nitrous acid is 4.50 ×
) .The acid dissociation constant of nitrous acid is 4.50 × 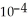 .
.
A) 3.487
B) 3.210
C) 13.86
D) 10.51
E) 4.562
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q33: Which of the following compounds will be
Q34: If a chemist wishes to prepare a
Q35: Sketch the titration curve for a strong
Q36: Calculate the solubility (in g/L)of silver chromate
Q40: Determine the molar solubility of Fe(OH)<sub>2 </sub>in
Q41: Which of the following is TRUE?<br>A) An
Q42: How many milliliters of 0.120 M NaOH
Q90: A 1.50 L buffer solution is 0.250
Q140: A 100.0 mL sample of 0.18 M
Q191: What is the pH at the equivalence