Multiple Choice
Calculate the percent ionization of nitrous acid in a solution that is 0.205 M in nitrous acid 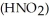 and 0.295 M in potassium nitrite (
and 0.295 M in potassium nitrite ( 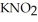 ) .The acid dissociation constant of nitrous acid is
) .The acid dissociation constant of nitrous acid is 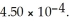
A) 59.0
B) 0.151
C) 17.1
D) 2.72 × 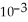
E) 3.508
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Calculate the pH of a solution formed
Q31: In which of the following solutions would
Q84: A solution contains 0.036 M Cu<sup>2+</sup> and
Q148: A 100.0 mL sample of 0.10 M
Q167: The molar solubility of CaF<sub>2</sub> is 2.15
Q170: Calculate the pH of a buffer that
Q171: You wish to prepare an HC<sub>2</sub>H<sub>3</sub>O<sub>2</sub> buffer
Q175: Calculate the K<sub>sp</sub> for zinc hydroxide if
Q176: Which one of the following statements is
Q185: A solution containing CaCl<sub>2</sub> is mixed with