Multiple Choice
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of 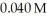 NaCN? Assume that the volumes of the solutions are additive and that Ka =
NaCN? Assume that the volumes of the solutions are additive and that Ka = 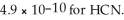
A) 2.0 × 10-10 M
B) 4.9 × 10-10 M
C) 1.2 × 10-9 M
D) 7.0 × 10-6 M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q71: Determine the molar solubility for Zn(OH)<sub>2</sub> in
Q72: What volume of 5.00 × 10<sup>-3</sup> M
Q74: Calculate the pH of a solution formed
Q75: Identify the compound that is acid-insoluble.<br>A) PbCl<sub>2</sub><br>B)
Q77: Animals will lick up ethylene glycol (antifreeze)due
Q78: Which of the following solutions is a
Q80: Match the following.
Q81: Which of the following is TRUE?<br>A) The
Q86: A 100.0 mL sample of 0.10 M
Q107: A 100.0 mL sample of 0.20 M