Multiple Choice
How many milliliters of 0.0839 M NaOH are required to titrate 25.0 mL of 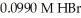 to the equivalence point?
to the equivalence point?
A) 29.5 mL
B) 0.332 mL
C) 4.57 mL
D) 0.208 mL
E) 21.2 mL
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q84: 0.10 M potassium chromate is slowly added
Q94: What is the pH of a buffer
Q120: Identify the compound that is in stalactites
Q179: Give the expression for the solubility product
Q181: A sample contains Ba<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub>,<sub> </sub>CdS,AgCl,NH<sub>4</sub>Cl,and ZnS.Identify the
Q182: Determine the solubility for CuC<sub>2</sub>O<sub>4</sub>(s)in pure water.K<sub>sp</sub>
Q183: Which of the following compounds will have
Q186: A buffer solution is 0.100 M in
Q187: The highest pH for an effective buffer
Q188: What is the pH of a solution