Multiple Choice
Two students are tasked with determining the milligrams of chloride in a simulated soil sample.Both students extract chloride into aqueous solution and perform triplicate titration with silver nitrate solution and dichlorofluorescein indicator.The students report the following results to their laboratory instructor. 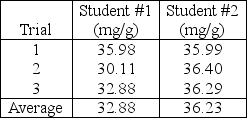 If the accepted value is 36.90 mg chloride/g sample,the laboratory instructor infers that student #1's work exhibits____________________ student #2.
If the accepted value is 36.90 mg chloride/g sample,the laboratory instructor infers that student #1's work exhibits____________________ student #2.
A) higher accuracy and higher precision than
B) higher accuracy and lower precision than
C) lower accuracy and lower precision than
D) lower accuracy and higher precision than
E) results impossible to differentiate from
Correct Answer:

Verified
Correct Answer:
Verified
Q10: An irregular solid with a mass of
Q11: Calculate the error for the molar mass
Q12: Calculate the mass of a heterogeneous mixture
Q13: For significant digits and calculations,the following statements
Q14: _ is a consistent error that can
Q15: Two students are tasked with determining the
Q16: When calculating the uncertainty associated with molar
Q17: Calculate the mass of a concrete slab
Q18: A mass of approximately 10 grams is
Q20: To the correct number of significant digits,what