For the Acid-Base Indicator Bromothymol Blue,the Protonated Form,HBB,is Yellow and the Deprotonated
Essay
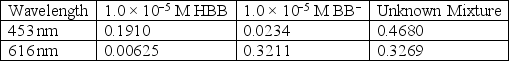
For the acid-base indicator bromothymol blue,the protonated form,HBB,is yellow and the deprotonated form,BB−,is blue.When both forms are present the indicator has a green color,the shade of green depends on the ratio of HBB:BB−.Use the information below to calculate the concentration of HBB and BB− for a green solution of bromothymol blue.
Correct Answer:

Verified
3.50 × 10−5 ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q10: For the plot of absorbance versus wavelength
Q11: Which is true for the relationship between
Q12: Which is NOT true for a Scatchard
Q13: For the UV-Vis absorption data below,calculate the
Q14: The stoichiometry for the complex MX<sub>n</sub> is
Q15: Sequential injection differs from flow injection by:<br>A)flow
Q16: An oxygen sensor is constructed from a
Q17: The method of continuous variation was applied
Q18: When a molecule absorbs a photon,the molecule
Q20: _enhances the fluorescence of immunoassays by a