Multiple Choice
Copper has two naturally occurring isotopes with atomic masses of 62.9296 u ( 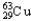 ) and 64.9278 u (
) and 64.9278 u ( 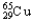 ) .The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
) .The atomic mass of copper is 63.546 u.What is the percent distribution of the isotopes?
A) 69.15% 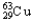 , 30.85%
, 30.85% 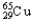
B) 30.85% 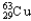 , 69.15%
, 69.15% 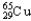
C) 1.08% 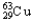 , 98.92%
, 98.92% 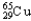
D) 98.92% 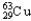 , 1.08%
, 1.08% 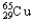
E) 99.03% 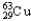 , 0.97%
, 0.97% 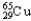
Correct Answer:

Verified
Correct Answer:
Verified
Q24: The natural distribution of the isotopes of
Q25: Which of the following is not a
Q26: Which of the following is correct for
Q27: Which of the following is correct for
Q28: Which of the following statements is false?<br>A)The
Q30: One of the main features of Dalton's
Q31: Which of the following statements is false?<br>A)Atoms
Q32: Consider the following figure. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3385/.jpg" alt="Consider
Q33: Which of the following elements are correctly
Q34: Which of the following is a correct