Multiple Choice
What is the half-reaction for the oxidation of sulfide ions in aqueous solution?
A) S2-(aq)  S(s) + 2 e-
S(s) + 2 e-
B) S-(aq) + e-  S2-(aq)
S2-(aq)
C) S(s) + 2 e-  S2-(aq)
S2-(aq)
D) SO32-(aq) + 6 H+(aq)  S(s) + 3 H2O(
S(s) + 3 H2O( 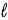 ) + 4 e-
) + 4 e-
E) SO42-(aq) + 8 H+(aq)  S(s) + 4 H2O(
S(s) + 4 H2O( 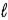 ) + 6 e-
) + 6 e-
Correct Answer:

Verified
Correct Answer:
Verified
Q21: Consider the following images.The reactions occurring in
Q22: Silver metal will not react with hydrochloric
Q23: Which of the following processes is a
Q24: What is the oxidizing agent in this
Q25: Consider the following beakers.In the beaker on
Q27: Match each term with its correct classification:<br>Proton
Q28: Reduction can be defined as...<br>A)a increase in
Q29: Balance the half-reaction in acidic solution and
Q30: In comparing acid-base neutralization reactions and redox
Q31: Identify the reduction half-reaction in the redox