Multiple Choice
What is the reducing agent in the reaction of hydrazine as a rocket fuel: N2H4( 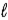 ) + O2(g)
) + O2(g)  N2(g) + 2 H2O(
N2(g) + 2 H2O( 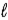 ) ?
) ?
A) N2H4
B) O2
C) N2
D) H2O
E) There is no reducing agent in this reaction
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: In balancing the half-reaction NO(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3385/.jpg"
Q36: Identify the oxidation half-reaction in the redox
Q37: Identify the oxidation half-reaction in the following
Q38: Which of the following statements is incorrect?<br>A)Acid-base
Q39: Which of the following is the correct
Q40: Which of the following is the half-reaction
Q41: When zinc is plated to iron,the zinc
Q42: Which of the following is a reduction
Q43: Examine the two beakers shown below. <img
Q45: What is the oxidation number of sulfur