Multiple Choice
Consider the following table of relative strengths of oxidizing and reducing agents: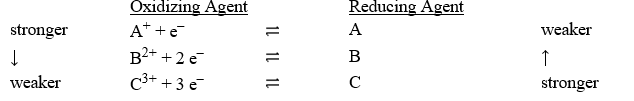
A) A < B < C
B) C < B < A
C) A+ < B2+ < C3+
D) B2+ < A+ < C3+
E) C3+ < B2+ < A+
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Which of the following cannot be a
Q9: In balancing the half-reaction SO<sub>4</sub><sup>2-</sup>(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3385/.jpg"
Q10: What happens to an elemental oxidizing agent
Q11: What evidence suggests that Na<sup>+</sup> ions are
Q12: What is the oxidation number of titanium
Q14: What is the balanced redox equation that
Q15: Oxidation-reduction reactions are also known as...<br>A)electron-transfer reactions<br>B)proton-transfer
Q16: Balance the following redox equation in acidic
Q17: In the reaction of carbon with oxygen,which
Q18: What is the oxidation number of chromium