Multiple Choice
Consider the following table of relative strengths of oxidizing and reducing agents: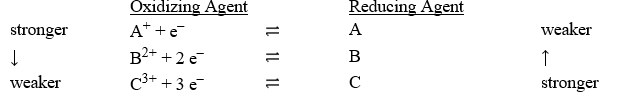
Which of the following is the correct net ionic equation for the redox reaction between A+ and C and the correct direction that is favored?
A) A+ + C  A + C3+ forward
A + C3+ forward
B) A+ + C  A + C3+ reverse
A + C3+ reverse
C) 3 A+ + C  3 A + C3+ forward
3 A + C3+ forward
D) 3 A+ + C  3 A + C3+ reverse
3 A + C3+ reverse
E) A+ + C + e-  A + C3+ + 3 e- forward
A + C3+ + 3 e- forward
Correct Answer:

Verified
Correct Answer:
Verified
Q27: Match each term with its correct classification:<br>Proton
Q28: Reduction can be defined as...<br>A)a increase in
Q29: Balance the half-reaction in acidic solution and
Q30: In comparing acid-base neutralization reactions and redox
Q31: Identify the reduction half-reaction in the redox
Q33: Which of the following statements is correct
Q34: How many electrons are transferred in the
Q35: In balancing the half-reaction NO(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3385/.jpg"
Q36: Identify the oxidation half-reaction in the redox
Q37: Identify the oxidation half-reaction in the following