Multiple Choice
Which of the following cannot act as a Brønsted-Lowry acid?
A) HCO3-(aq)
B) HOH( 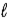 )
)
C) NH3(g)
D) CO32-(aq)
E) CH3OH( 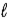 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Which of the following solutions is most
Q32: Which of the following conforms to the
Q33: A solution has a hydroxide ion concentration
Q34: A Brønsted-Lowry acid is defined as a(n):<br>A)proton
Q35: Consider a general comparison between acid-base and
Q37: What is the conjugate base of ammonia?<br>A)NH<sub>3</sub>(g)<br>B)NH<sub>4</sub><sup>+</sup>(aq)<br>C)NH<sub>4</sub>OH(aq)<br>D)NH<sub>2</sub><sup>-</sup>(aq)<br>E)OH<sup>-</sup>(aq)
Q38: What is the hydrogen ion concentration in
Q39: Which of the following is the correct
Q40: In Brønsted-Lowry acid-base reactions,which of the following
Q41: What is a Brønsted-Lowry base?<br>A)An electron pair