Multiple Choice
Consider the following image which depicts the reactants in an acid-base reaction.Atoms other than H are labeled with the element symbol. 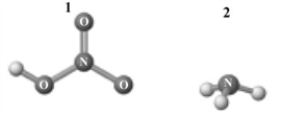
The products of this reaction are shown below. 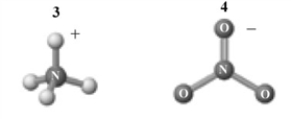
Which of the following is a correct interpretation of this reaction?
A) 1 is a Brønsted-Lowry acid.
B) 2 is a Brønsted-Lowry base.
C) The reaction is an acid-base neutralization.
D) 1 is also an Arrhenius acid.
E) All of the above are correct interpretations of this reaction.
Correct Answer:

Verified
Correct Answer:
Verified
Q14: Identify the conjugate acid base pairs in
Q15: Consider the following image. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3385/.jpg" alt="Consider
Q16: Which of the following substances is amphoteric?<br>A)H<sub>2</sub>O(
Q17: Which of the following is a Brønsted-Lowry
Q18: Which of the following properties is traditionally
Q20: The hydroxide ion concentration of a solution
Q21: Which pair below cannot have a Brønsted-Lowry
Q22: What is the conjugate acid of HPO<sub>4</sub><sup>2-</sup>(aq)?<br>A)H<sub>3</sub>PO<sub>4</sub>(aq)<br>B)HPO<sub>4</sub><sup>2-</sup>(aq)<br>C)H<sub>2</sub>PO<sub>4</sub><sup>-</sup>(aq)<br>D)PO<sub>4</sub><sup>3-</sup>(aq)<br>E)H<sub>3</sub>O<sup>+</sup>(aq)
Q23: A solution is made by dissolving 12.50
Q24: Which is the correct net ionic equation