Multiple Choice
Consider the following image which depicts the reactants in an acid-base reaction.Atoms other than H are labeled with the element symbol. 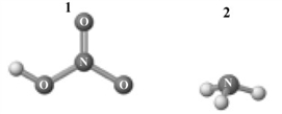 The products of this reaction are shown below.
The products of this reaction are shown below. 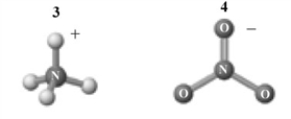 Which of the following is a correct interpretation of this reaction?
Which of the following is a correct interpretation of this reaction?
A) 1 and 2 are a conjugate acid-base pair.
B) 3 and 4are a conjugate acid-base pair.
C) 1 and 4 are a conjugate acid-base pair.
D) 2 and 4 are a conjugate acid-base pair.
E) 1 and 3 are a conjugate acid-base pair.
Correct Answer:

Verified
Correct Answer:
Verified
Q24: Which is the correct net ionic equation
Q25: Which of the following is a Lewis
Q26: A substance that can act as both
Q27: Consider the following generalized reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3385/.jpg"
Q28: Which of the following is a characteristic
Q30: Given the following relative base strengths,starting with
Q31: Which of the following solutions is most
Q32: Which of the following conforms to the
Q33: A solution has a hydroxide ion concentration
Q34: A Brønsted-Lowry acid is defined as a(n):<br>A)proton