Multiple Choice
What is the electron pair geometry shown in the following model? 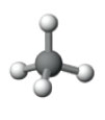
A) Tetrahedral
B) Angular (Bent)
C) Linear
D) Trigonal planar
E) Trigonal pyramid
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of the following has a trigonal
Q2: Which of the following is the best
Q4: Which of the following compounds is a
Q5: Which of the following has a(n)bent (angular)molecular
Q6: Which of the following is better classified
Q7: Which of the following wedge-and-dash diagrams best
Q8: Which of the following has a trigonal
Q9: Consider the following general wedge-and-dash diagram: <img
Q10: What is the molecular geometry surrounding the
Q11: Which of the following molecules is/are polar?<br>i.HF<br>ii.BH<sub>3</sub><br>iii.CBr<sub>2</sub>F<sub>2</sub><br>iv.PF<sub>3</sub><br>A)ii