Multiple Choice
Consider the following periodic table. 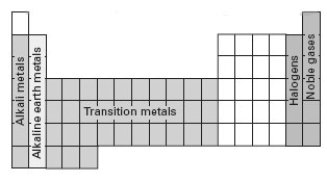 Which section represents elements whose electron configuration for the highest occupied energy level is ns2np5?
Which section represents elements whose electron configuration for the highest occupied energy level is ns2np5?
A) alkali metals
B) alkaline earth metals
C) transition metals
D) halogens
E) noble gases
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: List the atomic numbers of all atoms
Q11: How many orbitals are in the 5d
Q12: Which of the following is/are the major
Q13: The maximum number of electrons that can
Q14: In the third principal energy level,what is
Q16: Which of the following is the correct
Q17: Which of the following statements is/are correct?<br>i.Visible
Q18: What is the ground state electron configuration
Q19: The maximum number of electrons that can
Q20: Which of the following lists electron sublevels