Multiple Choice
Which of the following defines the percent yield for a reaction?
A) 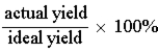
B) 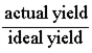
C) 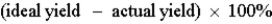
D) 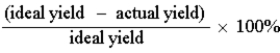
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Calculate the mass of Na<sub>2</sub>O that can
Q11: When C<sub>2</sub>H<sub>5</sub>Cl(g)is burned in oxygen,chlorine gas is
Q12: Consider the conversion diagram given below. <img
Q13: The metabolism of glucose can be represented
Q14: How many moles of C<sub>6</sub>H<sub>12</sub>O<sub>6</sub> are formed
Q16: Aqueous HCl is added to an aqueous
Q17: A typical candy bar contains 281 food
Q18: How many moles of oxygen are consumed
Q19: When one mole of gaseous hydrogen peroxide,H<sub>2</sub>O<sub>2</sub>,is
Q20: Convert 357 J to kcal.<br>A)1.49 kcal<br>B)1.49 ×