Multiple Choice
Consider the following thermochemical equation: CaO(s) + H2O( 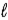 ) → Ca(OH) 2(s) ΔH = -66.5 kJ Which of the following statements is/are correct?
) → Ca(OH) 2(s) ΔH = -66.5 kJ Which of the following statements is/are correct?
i.The reaction is exothermic
ii.The reaction releases 66.5 kJ per gram of Ca(OH) 2(s) formed
iii.Alternatively,the ΔH term could be added to the product side of the equation
A) i only
B) ii only
C) i and ii
D) i and iii
E) i, ii, and iii
Correct Answer:

Verified
Correct Answer:
Verified
Q23: How many moles of water are produced
Q24: In a general chemistry laboratory experiment,a student
Q25: In the reaction 2 AgI + HgI<sub>2</sub>
Q26: In reacting aluminum carbonate with hydrochloric acid
Q27: If the reaction N<sub>2</sub> + 3 H<sub>2</sub>
Q29: The thermochemical equation describing the heat change
Q30: Consider the hypothetical reaction A + 2
Q31: In the reaction P<sub>4</sub> + 5 O<sub>2</sub>
Q32: A chemical reaction resulted in 1.376 g
Q33: In the combustion of natural gas according