Multiple Choice
What does the illustration represent? (Ref: P3e p.78) 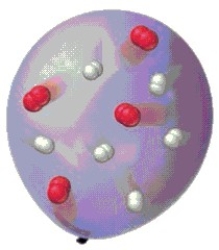
A) atomic element
B) molecular element
C) molecular compound
D) ionic compound
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Determine the empirical formula for a compound
Q78: Describe the difference between an atomic element
Q134: Write the formula for strontium nitride.<br>A)Sr<sub>3</sub>N<sub>2</sub><br>B)Sr(NO<sub>3</sub>)<sub>2</sub><br>C)SrN<br>D)Sr<sub>2</sub>N<sub>3</sub><br>E)Sr(NO<sub>2)2</sub>
Q135: The molecular weight of nitrous oxide (N<sub>2</sub>O),known
Q136: Which of the following is one possible
Q137: Calculate the moles of Fe in 16.0
Q138: Which statement is true?<br>A)All chemical bonds share
Q139: Determine the name for H<sub>2</sub>CO<sub>3</sub>.<br>A)carbonous acid<br>B)dihydrogen carbonate<br>C)carbonic
Q141: Write the formula for barium nitrite.<br>A)Ba<sub>3</sub>N<sub>2</sub><br>B)BaNO<sub>3</sub><br>C)BN<br>D)Ba(NO<sub>2</sub>)<sub>2</sub><br>E)B(NO<sub>2</sub>)<sub>3</sub>
Q143: How many moles of PCl<sub>3</sub> contain 3.68