Multiple Choice
Which container has the lowest pressure? All containers have same volume and temperature.Assume that the mass does not affect pressure.
A B C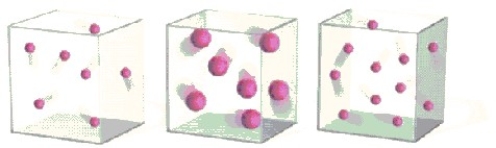
A) A
B) B
C) C
D) A and B
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q15: How many liters of hydrogen gas are
Q62: Determine the density of NH<sub>3</sub> gas at
Q72: Three identical flasks contain three different gases
Q107: Calculate the temperature,in K,of 2.20 moles of
Q109: A gas mixture consists of N<sub>2</sub>,O<sub>2</sub>,and Ne,where
Q110: Match the following.<br>-Dalton's Law<br>A)PV = nRT<br>B)V<sub>1</sub>/n<sub>1</sub> =
Q111: The mole fraction of argon in dry
Q112: The volume of 350.mL of gas at
Q113: A gas mixture contains CO,Ar and H<sub>2</sub>.What
Q168: How many liters of oxygen are needed