Multiple Choice
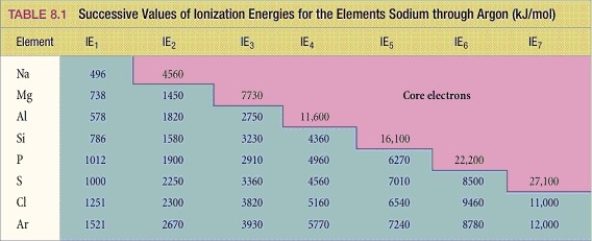
What period 3 element has the following ionization energies (all in kJ/mol) ?
IE1 = 1012 IE2 = 1900 IE3= 2910 IE4= 4960 IE5= 6270 IE6 = 22,200
A) Si
B) S
C) P
D) Cl
E) Mg
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following have their valence
Q17: Give the ground state electron configuration for
Q31: Of the following,which element has the highest
Q49: How many valence shell electrons does an
Q80: Of the following,which atom has the smallest
Q110: Write out the orbital diagram that represents
Q113: Choose the diamagnetic species from below.<br>A)Sn<sup>2</sup>⁺<br>B)Br<br>C)P<br>D)Cr<br>E)None of
Q115: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6106/.jpg" alt=" Refer to the
Q116: Give the ground state electron configuration for
Q117: Which reaction below represents the second ionization