Multiple Choice
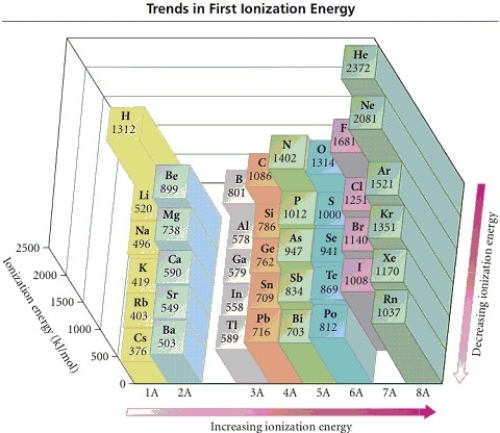
Refer to the figure.Place the following in order of increasing IE1.
K Ca Rb
A) Ca < K < Rb
B) Rb < Ca < K
C) Ca < Rb < K
D) Rb < K < Ca
E) K < Ca < Rb
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Give the complete electronic configuration for Mn.<br>A)
Q89: The condensed electron configuration of bromine,element 35,is
Q90: How many valence electrons does an atom
Q91: Match the following.<br>-valence electrons<br>A)0<br>B)2<br>C)electrons in the outermost
Q94: No two electrons can have the same
Q95: Choose the paramagnetic species from below.<br>A)Ti<sup>4+</sup><br>B)O<br>C)Ar<br>D)All of
Q96: Why does the size of the transition
Q97: What is the general valence-electron ground-state electron
Q98: Choose the valence orbital diagram that represents
Q114: Why is the first ionization energy of