Multiple Choice
Which of the following represent the Lewis structure for N?
A) 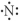
B) 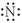
C) 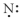
D) 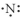
E) 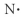
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q22: Which ionic compound would be expected to
Q85: Choose the best Lewis structure for BF<sub>3</sub>.<br>A)
Q86: Which of the following processes is exothermic?<br>A)the
Q88: Choose the bond below that is most
Q91: In the Lewis structure of CH<sub>3</sub>OH,how many
Q93: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6106/.jpg" alt=" Using the data
Q94: Choose the compound below that should have
Q95: Predict the formula of an ionic compound
Q130: Which of the following ionic compounds would
Q165: Describe a covalent bond.