Multiple Choice
Which of the following represent the Lewis structure for Cl?
A) 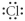
B) 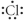
C) 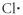
D) 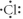
E) 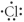
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Describe the difference between a pure covalent
Q51: The iodine atom in I<sub>2</sub> would be
Q52: Place the following in order of decreasing
Q76: Give the number of valence electrons for
Q78: Use Lewis theory to determine the chemical
Q79: Which of the following elements can form
Q82: Choose the best Lewis structure for SO<sub>4</sub><sup>2</sup>⁻.<br>A)
Q83: Which of the following reactions is associated
Q85: Choose the best Lewis structure for BF<sub>3</sub>.<br>A)
Q86: Which of the following processes is exothermic?<br>A)the