Multiple Choice
Consider the molecule below.Determine the molecular geometry at each of the 2 labeled carbons. 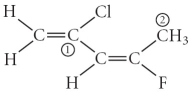
A) C1 = tetrahedral,C2 = linear
B) C1 = trigonal planar,C2 = bent
C) C1 = bent,C2 = trigonal planar
D) C1 = trigonal planar,C2 = tetrahedral
E) C1 = trigonal pyramidal,C2 = see-saw
Correct Answer:

Verified
Correct Answer:
Verified
Q46: What is the molecular geometry of TeCl<sub>4</sub>?<br>A)seesaw<br>B)square
Q68: How many of the following molecules are
Q69: Give the electron geometry,molecular geometry,and hybridization for
Q70: Determine the electron geometry,molecular geometry and polarity
Q71: Draw the Lewis structure for the molecule
Q74: How many electrons are present in the
Q76: Draw the Lewis structure for the molecule
Q77: Which molecule has unpaired electrons?<br>A)N<sub>2</sub><br>B)O<sub>2</sub><br>C)F<sub>2</sub><br>D)H<sub>2</sub>
Q126: Is it possible for a molecule to
Q150: The hybrid orbital set used by the