Multiple Choice
Consider the molecule below.Determine the molecular geometry at each of the 3 labeled atoms. 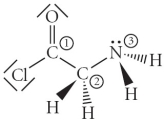
A) 1 = trigonal planar,2 = tetrahedral,3 = trigonal pyramidal
B) 1 = tetrahedral,2 = tetrahedral,3 = tetrahedral
C) 1 = trigonal planar,2 = tetrahedral,3 = tetrahedral
D) 1 = tetrahedral,2 = tetrahedral,3 = trigonal planar
E) 1 = trigonal planar,2 = trigonal pyramidal,3 = trigonal pyramidal
Correct Answer:

Verified
Correct Answer:
Verified
Q22: List the number of sigma bonds and
Q46: What is the molecular geometry of TeCl<sub>4</sub>?<br>A)seesaw<br>B)square
Q63: Give the approximate bond angle for a
Q65: Draw the Lewis structure for OF<sub>2</sub>.What is
Q66: Determine the electron geometry (eg),molecular geometry (mg),and
Q68: How many of the following molecules are
Q69: Give the electron geometry,molecular geometry,and hybridization for
Q78: The hybrid orbital set used by the
Q118: Using the VSEPR model, the molecular geometry
Q176: Using the VSEPR model, the electron-domain geometry