Multiple Choice
Assign the appropriate labels to the phase diagram shown below. 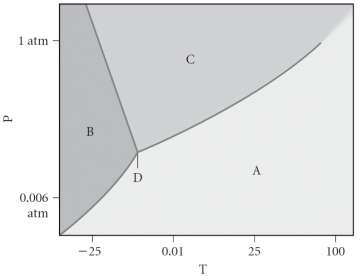
A) A = liquid,B = solid,C = gas,D = critical point
B) A = gas,B = solid,C = liquid,D = triple point
C) A = gas,B = liquid,C = solid,D = critical point
D) A = solid,B = gas,C = liquid,D = supercritical fluid
E) A = liquid,B = gas,C = solid,D = triple point
Correct Answer:

Verified
Correct Answer:
Verified
Q84: Match the following.<br>-LiI<br>A)dipole-dipole forces<br>B)dispersion forces<br>C)ion-dipole forces<br>D)ionic bond<br>E)hydrogen
Q85: In the phase diagram,what is the triple
Q86: How many atoms are present in a
Q87: The normal boiling point for H<sub>2</sub>Se<sub> </sub>is
Q89: What is the strongest type of intermolecular
Q91: Which of the following substances should have
Q92: Which of the following is considered a
Q93: Choose the pair of substances that are
Q108: In liquid propanol,CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>OH<sub> </sub> <sub> </sub>Which intermolecular
Q114: Nickel has a face-centered cubic structure and