Multiple Choice
Consider the phase diagram below.If the dashed line at 1 atm of pressure is followed from 100 to 500°C,what phase changes will occur (in order of increasing temperature) ? 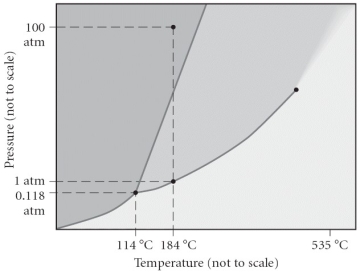
A) condensation,followed by vaporization
B) sublimation,followed by deposition
C) vaporization,followed by deposition
D) fusion,followed by vaporization
E) No phase change will occur under the conditions specified.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: What fraction of an atom is at
Q17: Choose the molecule or compound that exhibits
Q20: Which of the following statements is true?<br>A)Vapor
Q21: Ethanol (C<sub>2</sub>H<sub>5</sub>OH)melts at -114°C.The enthalpy of fusion
Q22: Which statement is correct?<br>A)The normal boiling point
Q23: Why is the ΔH<sub>vap</sub> higher than ΔH<sub>fus
Q24: Explain why the bolling point of water
Q47: How much energy is required to vaporize
Q121: Define viscosity.
Q123: How much energy must be removed from