Multiple Choice
Determine the radius of an Al atom (in pm) if the density of aluminum is 2.71 g/cm3.Aluminum crystallizes in a face centered cubic structure with an edge length of 2 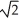
R.
A) 143 pm
B) 227 pm
C) 96 pm
D) 172 pm
E) 193 pm
Correct Answer:

Verified
Correct Answer:
Verified
Q31: Cesium has a radius of 272 pm
Q47: Calculate the vapor pressure of methanol at
Q49: Choose the substance with the lowest vapor
Q49: Identify the characteristics of a liquid.<br>A) indefinite
Q50: Match the following.<br>-CH<sub>3</sub>OH<br>A)dipole-dipole forces<br>B)dispersion forces<br>C)ion-dipole forces<br>D)ionic bond<br>E)hydrogen
Q51: What is the strongest type of intermolecular
Q55: Which has the highest boiling point?<br>A)CH<sub>3</sub>OH<br>B)CH<sub>3</sub>NH<sub>2</sub><br>C)CO<br>D)N<sub>2</sub>
Q57: Place the following compounds in order of
Q61: Which type of bonding does Sr form
Q73: Why do O,F and N,when bonded to