Multiple Choice
Vanadium crystallizes in a body-centered cubic structure and has an atomic radius of 131 pm.Determine the density of vanadium,if the edge length of a bcc structure is 4r/ 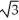
.
A) 3.06 g/cm3
B) 12.2 g/cm3
C) 6.11 g/cm3
D) 2.77 g/cm3
E) 8.46 g/cm3
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Which of the following is considered an
Q6: Place the following compounds in order of
Q8: What type of intermolecular force causes the
Q10: Choose the pair of substances that are
Q12: Choose the compound that exhibits hydrogen bonding
Q71: What is the edge length of a
Q103: How much energy must be removed from
Q104: Which is expected to have the largest
Q123: How much energy must be removed from
Q125: How much energy is required to heat