Multiple Choice
Express the equilibrium constant for the following reaction.
2 CH3Cl(g) + Cl2(g) ⇌ 2 CH2Cl2(g) + H2(g)
A) K = 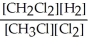
B) K = 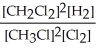
C) K = 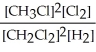
D) K = 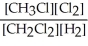
E) K = 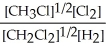
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q9: Consider the following reaction: 2 H<sub>2</sub>O(g)+ 2
Q40: Cyclohexane (C<sub>6</sub>H<sub>12</sub>)undergoes a molecular rearrangement in the
Q74: Determine the value of K<sub>c</sub> for the
Q80: What is △n for the following equation
Q81: The equilibrium constant is given for one
Q82: Which of the following statements are true?<br>A)Dynamic
Q84: What is △n for the following equation
Q87: Define reaction quotient.
Q88: What is △n for the following equation
Q122: Consider the following reaction at equilibrium.What effect