Multiple Choice
Express the equilibrium constant for the following reaction.
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
A) K = 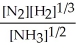
B) K = 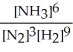
C) K = 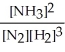
D) K = 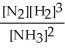
E) K = 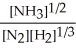
Correct Answer:

Verified
Correct Answer:
Verified
Q20: Consider the following reaction at equilibrium.What effect
Q60: The equilibrium constant is given for one
Q62: Express the equilibrium constant for the following
Q66: At 900.0 K,the equilibrium constant (K<sub>p</sub>)for the
Q67: The reaction below has a K<sub>c</sub> value
Q68: Consider the following reaction at equilibrium.What effect
Q69: Calculate P [NO]eq,if P [NOCl]<sub>eq</sub> = 0.33
Q70: Consider the following reaction at equilibrium.What effect
Q74: The equilibrium constant,K<sub>p</sub>,equals 3.40 at 25°C for
Q107: Consider the following reaction: NO(g)+ SO<sub>3</sub>(g)⇌ NO<sub>2</sub>(g)+