Multiple Choice
Express the equilibrium constant for the following reaction.
PCl5(g) ⇌ PCl3(g) + Cl2(g)
A) K = 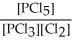
B) K = 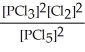
C) K = 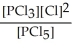
D) K = 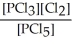
E) K = 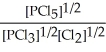
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Match the following.<br>-K << 1<br>A)Reaction will favor
Q34: Consider the following reaction,equilibrium concentrations,and equilibrium constant
Q35: The equilibrium constant (Kc)for the reaction N<sub>2</sub>O<sub>4</sub>(g)⇌
Q36: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q38: The reaction below has a K<sub>p</sub> value
Q40: An equilibrium mixture of CO,O<sub>2</sub> and CO<sub>2</sub>
Q41: The equilibrium constant is given for two
Q42: Determine the value of K<sub>p</sub> for the
Q100: Consider the following reaction: Xe(g)+ 2 F<sub>2</sub>(g)⇌
Q115: Can the K<sub>p</sub> and K<sub>c</sub> for a