Multiple Choice
Express the equilibrium constant for the following reaction.
H2(g) + Br2(g) ⇌ 2 HBr(g)
A) K = 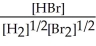
B) K = 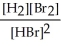
C) K = 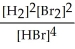
D) K = 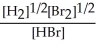
E) K = 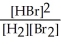
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Match the following.<br>-K << 1<br>A)Reaction will favor
Q12: At equilibrium in a 10.0 L vessel,there
Q13: Determine the value of K<sub>c</sub> for the
Q14: K<sub>c</sub> is 1.67 × 10<sup>20</sup> at 25°C
Q15: Match the following.<br>-Q < K<br>A)Reaction will favor
Q17: For which reaction will K<sub>p</sub> = K<sub>c</sub>?<br>A)S(s)+
Q18: Consider the following reaction at equilibrium.What effect
Q19: The equilibrium constant is given for one
Q20: What will happen to the following exothermic
Q21: Which of the following statements is true?<br>A)If