Multiple Choice
Calculate the cell potential for the following reaction that takes place in an electrochemical cell at 25°C.
Al(s) ∣ Al3+(aq,0.115 M) ∣∣ Al3+(aq,3.89 M) ∣ Al(s) 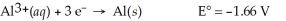
A) 1.66 V
B) 0.060 V
C) 0.00 V
D) 0.090 V
E) 0.030 V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q42: The electrolysis of molten AlCl<sub>3</sub> for 3.25
Q73: How many electrons are transferred in the
Q75: Using the following standard reduction potentials,<br>Fe<sup>3+</sup>(aq)+ e<sup>-</sup>
Q78: For the galvanic cell reaction,expressed below using
Q78: Determine the cell notation for the redox
Q79: Balance the following redox reaction if it
Q80: Determine which of the following pairs of
Q81: Which of the following is the weakest
Q107: How many grams of nickel metal are
Q110: For a galvanic cell that uses the